Introduction
Healthcare safety is often discussed in terms of protocols, training, and compliance. While these remain essential, hospitals globally are increasingly recognising another critical factor: system design.
In intravenous (IV) therapy, safety outcomes are not determined solely by how carefully a procedure is followed, but by how the IV system itself is designed. Open IV systems, multiple connection points, and repeated bedside handling can introduce variability and risk—even in well-run clinical environments.
This article explores how closed IV systems are reshaping conversations around infection control, nursing workflow, and procurement decisions.
Where Risk Enters Everyday IV Therapy
Across hospital audits and infection-control reviews, several recurring risk points appear consistently:
- Blood exposure during IV line access
- Multiple connection and disconnection steps
- Manual preparation under time pressure
- Dependence on perfect aseptic handling
These risks are not necessarily the result of poor practice. More often, they arise from system complexity—where multiple manual steps create more opportunities for variation.
In high-acuity environments, even small inconsistencies can accumulate into meaningful clinical risk.
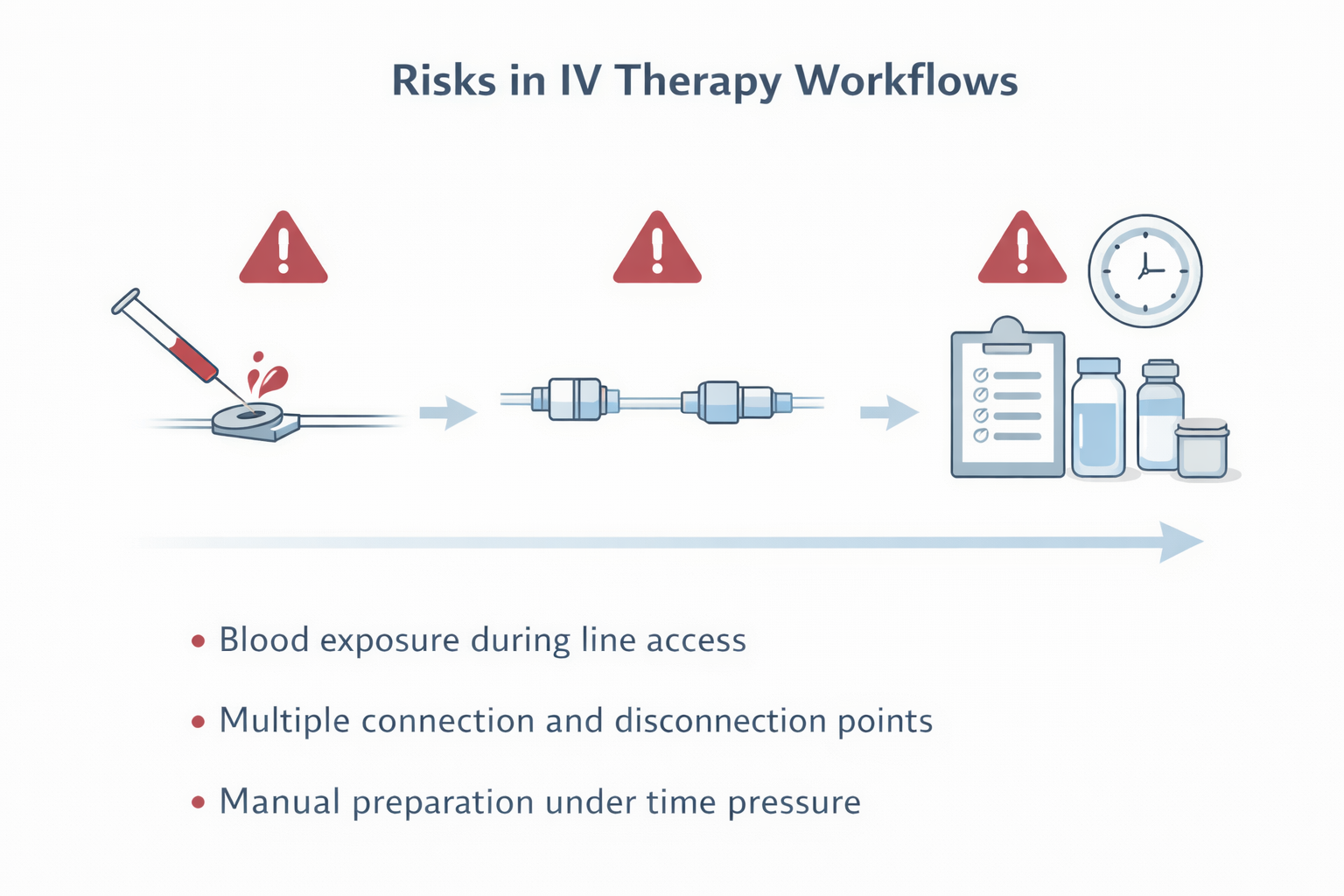
Illustration highlighting common points where variability and blood exposure can occur during routine IV therapy.
Open vs Closed IV Systems: A Design Difference
Open IV Systems
Open IV systems typically require:
- Air venting or external access to allow fluid flow
- Additional handling steps during setup and use
Each open access point represents a potential pathway for contamination or blood exposure.
Closed IV Systems
Closed IV systems are designed with:
- Sealed, needle-free access points
- Fewer handling and connection steps
By reducing exposure points and standardising access, closed systems aim to minimise variability in routine IV care.
The distinction is not about operator skill—but about how much the system depends on perfect execution every time.
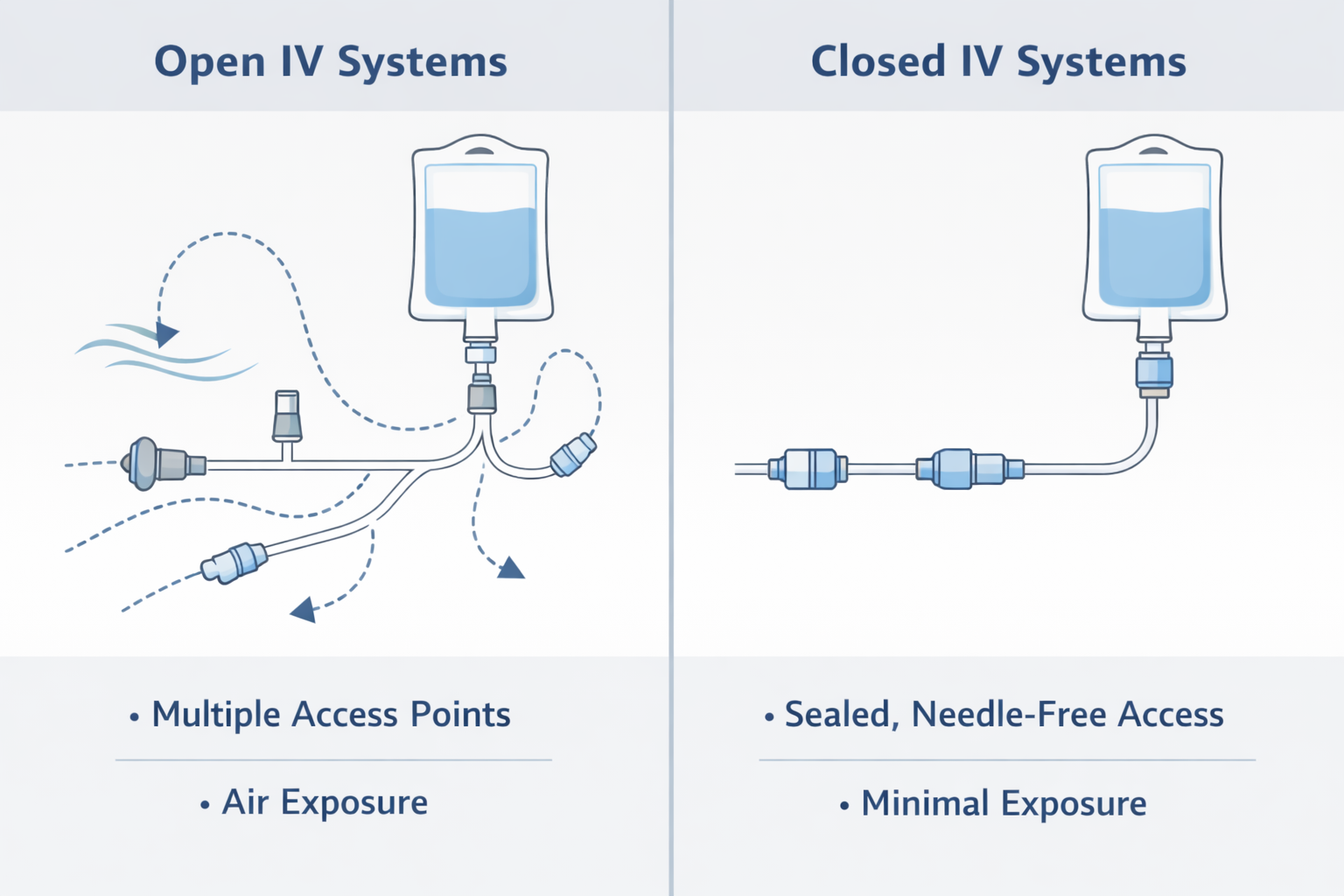
Conceptual illustration showing how open IV systems introduce multiple exposure points, while closed IV systems limit access through sealed connections.
Infection Risk and Clinical Outcomes
Multiple ICU studies and hospital audits have reported significant reductions in bloodstream infection rates following the adoption of closed IV systems.
Published data indicates:
- 70–85% reductions in bloodstream infections in certain ICU settings
- Infection rates falling from approximately 10.1 to 3.3 per 1,000 line days
While outcomes vary by setting, the trend is consistent: reducing open access points reduces infection risk.
For infection-control teams, this shifts the conversation from reactive investigation to proactive system design.
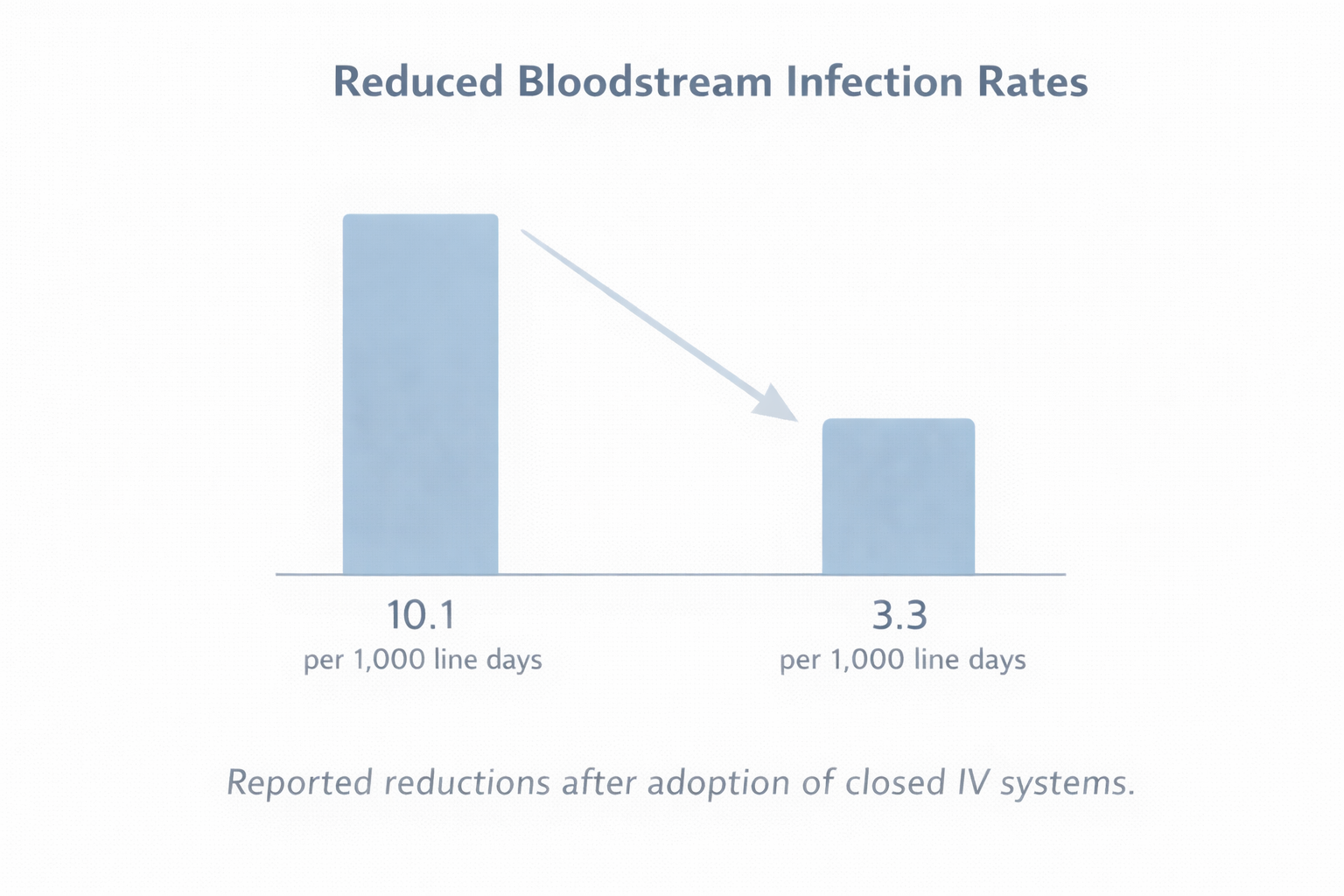
Reported reductions in bloodstream infection rates following the adoption of closed IV systems in ICU settings.
Implications for Nursing Workflow
IV therapy is a high-frequency task, often performed under time pressure and across multiple shifts. System design has a direct impact on nursing workload and safety.
Closed IV systems are associated with:
- Lower blood exposure risk during access
- Reduced cognitive load in high-acuity situations
- More consistent practice across shifts and teams
When systems require fewer manual steps, nurses spend less time managing equipment and more time focusing on patient care.
The Procurement Perspective: Moving Beyond Unit Price
For procurement teams, IV systems are often evaluated on unit cost. However, infection-related complications carry substantial downstream costs, including:
- Extended ICU stays
- Additional treatments and rework
- Staff exposure incidents
- Increased operational burden
Studies estimate the cost per bloodstream infection can range from €9,000 to €18,000, with some analyses suggesting hundreds of thousands of dollars in potential savings per 1,000 ICU patients when infection risk is reduced.
As a result, procurement decisions are increasingly framed around total cost of risk, not just purchase price.
System Design as a Safety Strategy
Modern IV therapy highlights a broader lesson in healthcare safety:
Design decisions increasingly influence clinical outcomes.
Reducing reliance on perfect human execution, minimising exposure points, and standardising workflows are powerful tools for improving safety at scale.
Closed IV systems represent one example of how thoughtful system design can support clinicians, protect staff, and improve patient outcomes.
Conclusion
The shift from open to closed IV systems reflects a broader evolution in healthcare thinking—from focusing solely on protocols to examining how systems shape everyday practice.
For clinicians, nurses, and procurement leaders alike, the question is no longer just how care is delivered—but how the system itself supports safe, consistent delivery.
Frequently Asked Questions
What is a closed IV system?
A closed IV system minimizes open access points during IV therapy, reducing blood exposure and
contamination risk during line access.
Do closed IV systems help reduce infection risk?
Evidence suggests that limiting open handling steps supports infection-control efforts by reducing exposure events and procedural variability.